

Biological Significance of Calcium Calcium is an essential element in the human body. Calcium has two valence electrons and a very low electronegativity of 1. It has an electron configuration of Ar2s 2. Let me tell you how this Interactive Periodic Table will help you in your studies.ġ). It is in the fourth row of the periodic table, beneath magnesium.
Calcium number on periodic table free#
Free Gift for you: Interactive Periodic Table It is an essential constituent of leaves, bones, teeth. In this way, the elements of the same group show similar chemical properties and they also have the same number of valence electrons. Calcium, a metallic element, is fifth in abundance in the earths crust, of which it forms more than 3. In the Modern Periodic Table, calcium (atomic number 20) is surrounded by elements with atomic numbers 12,19,21, and 38.
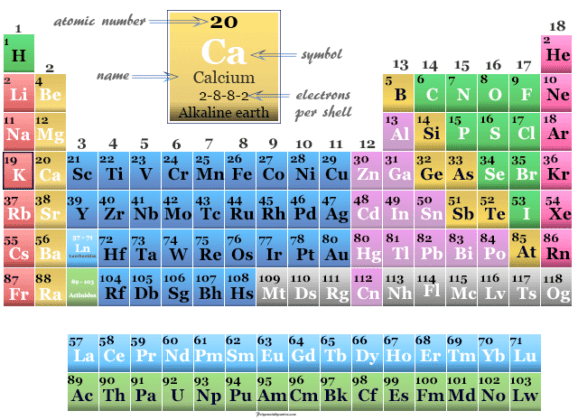
They are soft and can be cut easily with a kitchen knife.Īlso all the elements of group 1 have one valence electron.Īll the elements of group 18 are chemically inert (that means they do not easily react with other elements).Īnd all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell). The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.Īll the elements of group 1 are highly reactive to water. Calcium (Ca, atomic number 20) is located in group just below magnesium (Mg, atomic number 12) in the peri periodic. Atomic Mass Average: 40.078Boiling Point: 1757K 1484C 2703FCoefficient of lineal thermal expansion/K-1: 22EConductivity.

There are total 18 vertical columns on periodic table. In adults, calcium deficiency is strongly related to increasing severity of osteoporosis. They are used to measure calcium absorption mainly in women and children. Calcium is a solid at standard temperature and pressure.Groups are the vertical columns on the periodic table. Calcium isotopes (mainly Ca-42, Ca-44, Ca-46 and Ca-48) are used extensively in clinical research and mainly in nutritional studies. Calcium reacts strongly with water to produce Hydrogen gas and Calcium Hydroxide and strongly with acid to produce Calcium salts. Calcium, symbol Ca, is an alkaline earth metal in the second column of the periodic table. It is a structural material, being present in cell walls, bones, teeth.
Calcium number on periodic table series#
Calcium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis. It is one of the six bulk elements, being the 5th most common element in the human body. Properties Calcium is a more reactive alkali earth metal than Magnesium but less reactive than Strontium. Calcium ions have lost two electrons to become positively charged. An atom of Calcium has only 2 electrons in its outer shell. Atomic Structure The most stable isotope of Calcium has 20 neutrons in its nucleus giving it an atomic mass of 40. All living organisms (in fact, even dead ones) have and need calcium for survival. Calcium is the fifth most abundant element by mass (3.4) in both the Earth's crust and in seawater. It is a group 2 metal, also known as an alkaline-earth metal, and no populated d-orbital electrons. Calcium atoms join together in a giant metallic structure. Calcium is the 20th element in the periodic table. Chalkboard with description of periodic table notation for calcium. MeaningĬalcium is a Group 2 element, on the Periodic Table, with 20 protons in the nucleus.Ībout Calcium Molecular Structure Calcium has the chemical symbol Ca. A 2 dimensional representation of the Bohr Model of a Calcium-40 isotope with 20 protons and 20 neutrons in the nucleus and 2 electrons in the first shell, 8 in the second, 8 in the third and 2 in the outer shell.


 0 kommentar(er)
0 kommentar(er)
